What is Passivation reaction?

Passivation reaction is a process in which the surface of a metal is chemically treated to form a passive oxide layer, making the metal more corrosion-resistant. This typically involves exposing the metal to an environment containing oxygen, allowing it to react and form an oxide film.
The most typical passivation reaction occurs on the surface of steel. In the atmosphere, iron reacts with oxygen to form iron oxide, commonly known as rust (Fe2O3 or Fe3O4). The iron oxide film can form on the metal surface, acting as a protective layer to slow down further oxidation reactions, thus enhancing the metal's corrosion resistance.
Some metals, such as aluminum, chromium, and stainless steel, can also undergo passivation through specific chemical treatments. This process often involves immersing the metal in an acidic solution to create a dense oxide layer on the surface, improving the metal's corrosion resistance.
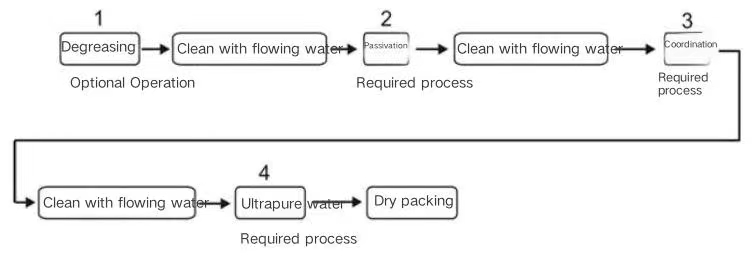
Benefits of passivation in machining products include:
Corrosion Resistance: The oxide layer formed during passivation covers the metal surface, preventing the metal from reacting with oxygen, water, etc., and thus enhancing the corrosion resistance of metal parts.
Increased Surface Hardness: Passivation can create a relatively hard oxide layer, improving the hardness of the metal surface and increasing the wear resistance of parts.
Improved Surface Smoothness: Passivation treatment often leads to improved surface smoothness, which is beneficial for parts with high surface quality requirements.
Enhanced Lubrication: The passivated surface is generally smoother, contributing to improved friction performance and enhanced lubrication effects.
Aesthetic Improvement: Passivation can change the color of the metal surface, making it more visually appealing, which is important for applications with high aesthetic requirements.
Reduced Electrical Conductivity: The passivation layer is typically an insulator, reducing the electrical conductivity of metal parts, suitable for special applications with conductivity requirements.